“Theoretical Investigation of Lactide Ring-Opening Polymerization Induced by a Dinuclear Indium Catalyst”
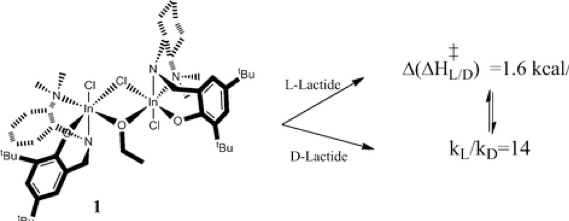
A DFT study of the ring-opening polymerization of lactide (LA) induced by a dinuclear indium catalyst supported by a chiral diamino phenoxy ligand, [(NNHO)InCl]2(μ-Cl)(μ-OEt) (1), is reported. The nature of the active catalyst, mononuclear vs dinuclear, was investigated and was shown to be dinuclear because of the high energetic cost of its
dissociation. The selectivity of the system was investigated for the polymerization of LA with the dinuclear (R,R/R,R)-1
catalyst. In complete agreement with experimental results we observed that (1) selectivity is controlled by the nucleophilic addition of LA to the alcoholate, resulting in the chain-end control of polymerization, (2) a slight kinetic preference for the polymerization of L-LA over D-LA is found that translates to a krel value of ∼14, which is identical with the experimental value, and (3) when rac-LA is used, no clear preference for D- vs L-LA insertion is found, leading to isotactic PLA.