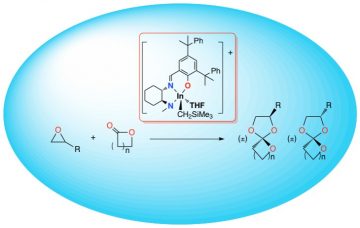
We report a cationic indium catalyst for the conversion of epoxides and lactones to spiro-orthoesters, a family of expanding monomers. The cationic indium alkyl complexes [(NNiO)In(CH2SiMe3)(S)][B(3,5-(CF3)2C6H3)4] (2•S; S = OEt2 or THF) were synthesized and fully characterized. The reaction of ε-caprolactone and 1,2-epoxy-7-octene forms 2-(hex-5-en-1-yl)-1,4,6-trioxaspiro[4.6]undecane (SOE1) with full conversion of both components. We can expand the substrate scope to 5 and 6 membered lactones as well as a number of functionalized epoxides. The further reactivity of the SOEs is discussed.