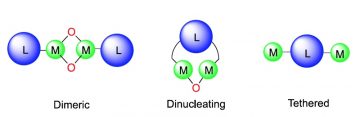
Poly(lactic acid) (PLA) is by far the most commonly produced biodegradable polyester. Metal-mediated ring opening polymerization of lactide is the most studied route for the synthesis of PLA, with hundreds of catalysts reported to date. Discrete metal initiators are comprised of an electropositive metal center, an ancillary ligand, and an initiating group such as an amide or an alkoxide. This difference in polarity between the electrophilic metal centers and the nucleophlic initiators often leads to catalyst aggregation. Partially due to this phenomenon, bimetallic catalysts, either dimeric, tethered, or dinucleating, are gaining attention as catalysts. The involvement of two metals in each of these motifs can affect the mechanism, and thus the activity, of the catalytic systems differently. This review is divided into three main segments: A) Dinuclear or dimeric species arising from aggregation of two discrete metal centers through bridging ligands; B) Tethered species involving two non-bridged metal centers on the same ligand architecture that can react independently and C) Dinucleating catalysts involving a multidentate ligand platform bound to two different metals, which are also bridged by a secondary ligand and can react in tandem. This review explores the structure-function relationship for each of these motifs by examining the effect of ligand design on various modes of bimetallic cooperativity in the ring opening polymerization mechanism of lactide.